Cytochromes P450 (CYPs) are a very large family of heme-thiolate proteins that catalyze numerous reactions between organic substrates and oxygen. For example, CYPs are able to activate C-H bonds in hydrocarbon groups and functionalize them by insertion of oxygen.
We are currently studying P450cam (also known as CYP101A1), a bacterial camphor hydroxylase, in two ways:
1) when this enzyme has been active (hydroxylating camphor) and runs out of oxygen, it starts reducing camphor to borneol. We discovered this process and continue to study its mechanism and function in the soil bacteria that have P450cam (1).
2) We have prepared families of P450cam mutants by random mutagenesis and have found variants with interesting catalytic abilities. For example, we found a group of mutants that dehalogenates 3-chloroindole (2). Such enzymes could one day be useful in the metabolism of persistent pollutants, such as the insecticide endosulfan.
Examples of recent projects:
1. We studied the chemotaxis of the soil bacterium Pseudomonas putida (the strain that harbors P450cam) and found different chemotactic behaviors with camphor, depending on oxygenation levels and the integrity of P450cam. Balaraman, P. and Plettner E. (2019) Biochim. Biophys. Acta – General Subjects 1863: 304-312.
2. Kammoonah, S. et al. (2018) Biochim. Biophys. Acta – Proteins and Proteomics 1866: 68-79.
We are currently studying P450cam (also known as CYP101A1), a bacterial camphor hydroxylase, in two ways:
1) when this enzyme has been active (hydroxylating camphor) and runs out of oxygen, it starts reducing camphor to borneol. We discovered this process and continue to study its mechanism and function in the soil bacteria that have P450cam (1).
2) We have prepared families of P450cam mutants by random mutagenesis and have found variants with interesting catalytic abilities. For example, we found a group of mutants that dehalogenates 3-chloroindole (2). Such enzymes could one day be useful in the metabolism of persistent pollutants, such as the insecticide endosulfan.
Examples of recent projects:
1. We studied the chemotaxis of the soil bacterium Pseudomonas putida (the strain that harbors P450cam) and found different chemotactic behaviors with camphor, depending on oxygenation levels and the integrity of P450cam. Balaraman, P. and Plettner E. (2019) Biochim. Biophys. Acta – General Subjects 1863: 304-312.
2. Kammoonah, S. et al. (2018) Biochim. Biophys. Acta – Proteins and Proteomics 1866: 68-79.
Honey bee health and pheromones
Honey bees (Apis mellifera L.) play an essential role in food production, the environment and our economies. Not only do bees produce honey and wax, but they pollinate approximately half of the crops we rely on for our survival.
Honey bees are eusocial insects, which means that they have overlapping generations, a division of labor and distinct reproductive and non-reproductive castes. The only female honey bee that reproduces is the queen; the workers take over all non-reproductive tasks in the colony, such as nursing of the brood, cleaning, building comb, guarding and foraging for food outside of the colony. Workers go through a regime of maturation in their tasks, which begins with work around the brood nest, then proceeds to nursing brood and finally ends with work outside of the nest. To ensure that the ratio of bees working inside of the nest and foraging outside is consistent with the needs of the entire colony, forager bees produce a pheromone (ethyl oleate) which controls the rate at which nurse bees mature into foragers. We have studied the biosynthesis of this pheromone (1) and continue to study its transport and chemoreception.
Honey bees are afflicted by multiple diseases and parasites, and the varroa mite (Varroa destructor Anderson and Trueman) is at the top of the list. We have studied compounds that alter the host choice of varroa mites (2) or that paralyze and eventually kill the mites, with no harm to the bees (3).
Examples of projects:
1. Castillo, C. et al. (2012) Insect Biochem. Mol. Biol. 42: 404 – 416.
2. Pinnelli, G. R. et al. (2016) J. Agric. Food Chem. 64: 8653 8658.
3. Testing of a selective acaricide against the Varroa mite.
Honey bees are eusocial insects, which means that they have overlapping generations, a division of labor and distinct reproductive and non-reproductive castes. The only female honey bee that reproduces is the queen; the workers take over all non-reproductive tasks in the colony, such as nursing of the brood, cleaning, building comb, guarding and foraging for food outside of the colony. Workers go through a regime of maturation in their tasks, which begins with work around the brood nest, then proceeds to nursing brood and finally ends with work outside of the nest. To ensure that the ratio of bees working inside of the nest and foraging outside is consistent with the needs of the entire colony, forager bees produce a pheromone (ethyl oleate) which controls the rate at which nurse bees mature into foragers. We have studied the biosynthesis of this pheromone (1) and continue to study its transport and chemoreception.
Honey bees are afflicted by multiple diseases and parasites, and the varroa mite (Varroa destructor Anderson and Trueman) is at the top of the list. We have studied compounds that alter the host choice of varroa mites (2) or that paralyze and eventually kill the mites, with no harm to the bees (3).
Examples of projects:
1. Castillo, C. et al. (2012) Insect Biochem. Mol. Biol. 42: 404 – 416.
2. Pinnelli, G. R. et al. (2016) J. Agric. Food Chem. 64: 8653 8658.
3. Testing of a selective acaricide against the Varroa mite.
Insect chemoreception
Insects use their chemical senses to detect signals emitted by members of their species (pheromones), by their hosts or even their predators. One fascinating aspect of chemical communication of insects is that they can distinguish tiny differences between compounds. For example, gypsy moths (Lymantria dispar) distinguish the enantiomers of their pheromone, disparlure, very accurately.
We study the molecular components of the chemical senses with the overarching goal of understanding their function, as it relates to insect survival. We also use this knowledge to develop compounds and strategies that disrupt the behaviors of pest insects, mediated by chemical signals.
Examples of recent projects:
1. Determination of the structure of pheromone-binding protein 1 of the European gypsy moth (L. dispar dispar).
2. Development of a short synthesis of the gypsy moth pheromone (disparlure) and study of the disparlure enantiomer distinction by gypsy moth pheromone-binding proteins. Pinnelli, G. R., Terrado, M. et al. (2019) Eur. J. Org. Chem. Doi: 10.1002/ejoc.201901164
We study the molecular components of the chemical senses with the overarching goal of understanding their function, as it relates to insect survival. We also use this knowledge to develop compounds and strategies that disrupt the behaviors of pest insects, mediated by chemical signals.
Examples of recent projects:
1. Determination of the structure of pheromone-binding protein 1 of the European gypsy moth (L. dispar dispar).
2. Development of a short synthesis of the gypsy moth pheromone (disparlure) and study of the disparlure enantiomer distinction by gypsy moth pheromone-binding proteins. Pinnelli, G. R., Terrado, M. et al. (2019) Eur. J. Org. Chem. Doi: 10.1002/ejoc.201901164
Synthesis of bioactive compounds
and molecular probes
and molecular probes
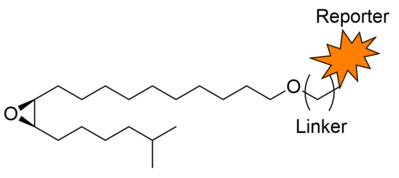
Synthesis is an important tool that helps us assemble the pheromones, general odorants, feeding deterrents, analogs and molecular probes we use in our projects. Molecular probes are particularly important, to identify, image and study the target sites of various bioactive molecules.
Examples of projects that have utilized synthesis:
1. Yu, Y., Plettner, E. (2013) Bioorg. Med. Chem. 21: 1811–1822.
2. Pinnelli, G. R. et al. (2016) J. Agric. Food Chem. 64: 8653 8658.
3. Pinnelli, G. R., Terrado, M. et al. (2019) Eur. J. Org. Chem. Doi: 10.1002/ejoc.201901164
Examples of projects that have utilized synthesis:
1. Yu, Y., Plettner, E. (2013) Bioorg. Med. Chem. 21: 1811–1822.
2. Pinnelli, G. R. et al. (2016) J. Agric. Food Chem. 64: 8653 8658.
3. Pinnelli, G. R., Terrado, M. et al. (2019) Eur. J. Org. Chem. Doi: 10.1002/ejoc.201901164

